ELTE Research Centre for the Humanities | 1097 Budapest, Tóth Kálmán utca 4. | HU15854939
- Details
- Category: Laboratory
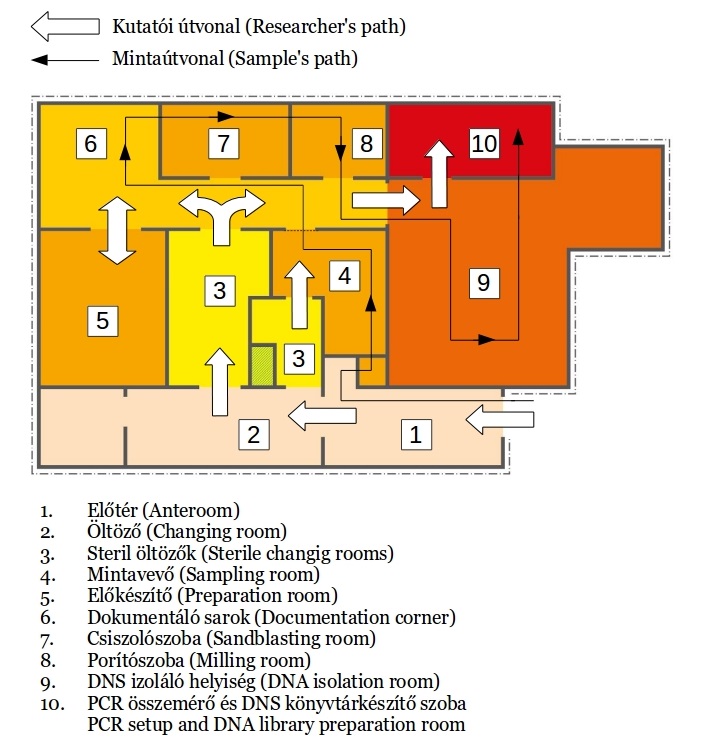 While most modern DNA molecules exist as long intact strands, ancient DNA is typically damaged, enriched in deaminations, and fragmented into small pieces. The primary objective of an ancient DNA facility is to prevent contamination with modern nucleic acids. This is especially critical for the analysis of human samples, where the risk of contamination by laboratory personnel, museum staff, or archaeological and anthropological colleagues is particularly high. Strict separation between pre- and post-PCR work phases is crucial to prevent the transfer of modern DNA molecules from the post-PCR to the pre-PCR laboratory. To mitigate this risk, the two laboratories are located on separate floors.
While most modern DNA molecules exist as long intact strands, ancient DNA is typically damaged, enriched in deaminations, and fragmented into small pieces. The primary objective of an ancient DNA facility is to prevent contamination with modern nucleic acids. This is especially critical for the analysis of human samples, where the risk of contamination by laboratory personnel, museum staff, or archaeological and anthropological colleagues is particularly high. Strict separation between pre- and post-PCR work phases is crucial to prevent the transfer of modern DNA molecules from the post-PCR to the pre-PCR laboratory. To mitigate this risk, the two laboratories are located on separate floors.
The main parts of the laboratory:
 Ancient DNA clean rooms (pre-PCR laboratory)
Ancient DNA clean rooms (pre-PCR laboratory)
Access to the ancient DNA clean laboratory involves passing through three changing rooms, where researchers change into specialized attire including bodysuits, gloves, specific shoes, face masks, and hairnets. The storage room also houses laboratory supplies. Every item entering the clean rooms undergoes thorough washing with bleach (sodium hypochlorite - NaClO) followed by UV-C irradiation.
 No modern DNA work is conducted in the pre-PCR laboratory. Work surfaces, reagents, and equipment are routinely irradiated and cleaned with bleach to eliminate any modern DNA contamination. The sample preparation room features a dedicated isolated cabin for the primary cleaning, cutting, and polishing of samples, along with a vacuum system. The cleanrooms of the laboratory are maintained at positive air pressure, ensuring a consistent pressure gradient throughout the facility, with the highest pressure in the DNA extraction room. This room is equipped with a laminar flow hood for conducting DNA extraction procedures. Additionally, a separate preparatory room is equipped with a fume hood for handling reagents, especially those that may be toxic. We utilize HPLC-grade water for reactions and ultrapure water for cleaning purposes.
No modern DNA work is conducted in the pre-PCR laboratory. Work surfaces, reagents, and equipment are routinely irradiated and cleaned with bleach to eliminate any modern DNA contamination. The sample preparation room features a dedicated isolated cabin for the primary cleaning, cutting, and polishing of samples, along with a vacuum system. The cleanrooms of the laboratory are maintained at positive air pressure, ensuring a consistent pressure gradient throughout the facility, with the highest pressure in the DNA extraction room. This room is equipped with a laminar flow hood for conducting DNA extraction procedures. Additionally, a separate preparatory room is equipped with a fume hood for handling reagents, especially those that may be toxic. We utilize HPLC-grade water for reactions and ultrapure water for cleaning purposes.- Post-PCR laboratories
The post-PCR laboratory consists of two departments. One department comprises a semi-clean room for DNA library handling and a dedicated sequencing roo
 m, while the other sector is utilized for post-PCR analyses. The latter is equipped with thermocyclers, electrophoresis equipment, and gel documentation systems. PCR reactions set up in the clean rooms are transferred to the post-PCR lab for amplification and preparation for downstream analyses. Additionally, the post-PCR laboratory features an extra clean bench specifically designated for hybridization capture reactions. This section of the lab is also utilized for the extraction, amplification, and sequencing of modern DNA samples. To prevent cross-contamination, workflow and sample treatment are strictly segregated between pre-PCR and post-PCR stages. Researchers at the Institute of Archaeogenomics are capable of processing and sequencing highly degraded DNA samples (refer to Chapter Methods for more details).
m, while the other sector is utilized for post-PCR analyses. The latter is equipped with thermocyclers, electrophoresis equipment, and gel documentation systems. PCR reactions set up in the clean rooms are transferred to the post-PCR lab for amplification and preparation for downstream analyses. Additionally, the post-PCR laboratory features an extra clean bench specifically designated for hybridization capture reactions. This section of the lab is also utilized for the extraction, amplification, and sequencing of modern DNA samples. To prevent cross-contamination, workflow and sample treatment are strictly segregated between pre-PCR and post-PCR stages. Researchers at the Institute of Archaeogenomics are capable of processing and sequencing highly degraded DNA samples (refer to Chapter Methods for more details).
Selection of Laboratory Equipment:

- Sandblasting tools
- Retsch mixer mill
- Laminar flow cabinets and PCR workstations
- PCR equipment, centrifuges, rotators, hybridization chamber, Eppendorf thermal cyclers
- Merck Millipore vacuum filtration system
- Real-time PCR system 7500 (Applied Biosystem)

- Illumina MiSeq sequencer
- TapeStation 4200 System (Agilent Genomics)
- Qubit fluorometer
- Vision Express gel documentation system
- Beckmann Coulter Biomek i5 robots
If you are interested in collaborating with us, please contact our laboratory chief for further details.
- Details
- Category: Laboratory
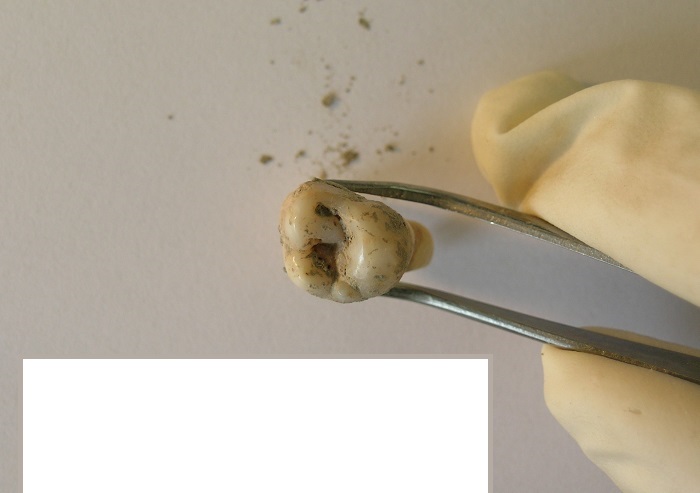
 The most suitable material for archaeogenetic investigations is teeth and those bones, which have thick cortical tissue parts (compacta). These skeletal remains have the best ancient DNA preservation for our analyses. Recent results show that the petrosal parts of the temporal bones preserve particularly large amount of human DNA compared to other skeletal elements.
The most suitable material for archaeogenetic investigations is teeth and those bones, which have thick cortical tissue parts (compacta). These skeletal remains have the best ancient DNA preservation for our analyses. Recent results show that the petrosal parts of the temporal bones preserve particularly large amount of human DNA compared to other skeletal elements.
- Our primary target material is the petrous part of the temporal bones (pars petrosa ossis temporalis)
- The second examined materials are intact teeth from which molars are favored.
- Thirdly, long bones are sampled, which have relatively thick compacta, because this part keeps the aDNA in the best quality. Such bones include the femur, tibia, and humerus, which have a robust cortical substance.
Ancient DNA in the organic remains is exposed to effects of heat, thermal changes, humidity, and other environmental effects. Moreover, the post-excavation history of a specimen significantly influences the ancient DNA preservation and possible endogenous DNA content of the sample. In order to avoid modern DNA contamination of the studied specimens, in-situ sampling on the archaeological excavation is considered an ideal approach.
- Details
- Category: Laboratory
 Upon entering our clean pre-PCR laboratory, each bone sample receives a unique identifier, is registered, and photographed. A special isolated cabin is used for the primary mechanical cleaning of the bones. The entire cabin is regularly bleached and exposed to UV-C irradiation before and after the scrapings. The samples are also exposed to UV-C irradiation from all sides upon entering the lab. The surfaces of the bone samples are removed using a polisher machine. A mobile vacuum cleaner continuously removes the bone powder by-product during polishing. Tooth samples are cleaned using UV-C irradiation and a sandblaster machine. The cleaned samples, which have been subsequently irradiated with UV-C, are placed into sterile bags until further analyses.
Upon entering our clean pre-PCR laboratory, each bone sample receives a unique identifier, is registered, and photographed. A special isolated cabin is used for the primary mechanical cleaning of the bones. The entire cabin is regularly bleached and exposed to UV-C irradiation before and after the scrapings. The samples are also exposed to UV-C irradiation from all sides upon entering the lab. The surfaces of the bone samples are removed using a polisher machine. A mobile vacuum cleaner continuously removes the bone powder by-product during polishing. Tooth samples are cleaned using UV-C irradiation and a sandblaster machine. The cleaned samples, which have been subsequently irradiated with UV-C, are placed into sterile bags until further analyses.
The samples are ground into a fine powder using a mixer mill (Retsch), crushing in ball milling mills, or by drilling bone powder. The resulting powder is measured in a sterile box and then stored in sterile tubes at 4°C until use. Each batch of samples has a negative control taken from the milling and later from the DNA extraction. The negative controls are processed parallel with the bone samples.
The DNA extraction takes place in clean laboratory conditions, under a laminar flow box in a separate room. Several extraction methods are applied depending on the state of the sample and the purpose of the analysis. The DNA extraction workflow has been automated in Biomek workstations, making it easier and faster to process samples. The DNA extracts, marked by specific ID numbers, are stored in the freezer. Each series uses separate isolation negative controls, which are also treated in parallel with the sample. This contains only the solutions used in the purification process.
From DNA extractions, DNA libraries are prepared manually or using robotic technology. During DNA library preparation, DNA is chemically repaired, and samples are barcoded with individual adapters. After amplification, the samples are prepared for next-generation sequencing (NGS), which is performed in-house using the Illumina MiSeq Platform. Depending on our research objectives, DNA regions are selected (whole mitochondrial genome, single nucleotide polymorphisms) from the samples using the method of target capture, or random “shutgun” sequencing is performed.
Autosomal DNA
 In diploid human cells, autosomes consist of 22 pairs of chromosomes, each containing two variants of an allele or marker. These markers are present in diploid form, meaning there are maternal and paternal pairs; however, they may carry different alleles and variants of the same gene. Autosomal DNA examination is suitable for determining familial relationships through bloodlines. The likelihood of establishing a direct familial relationship increases with the number of markers tested and the size of the sections examined. The autosomes are commonly used in forensic science for the identification and assignment of skeletal elements in disturbed burial sites, reconstruction of historic genealogies, and families.
In diploid human cells, autosomes consist of 22 pairs of chromosomes, each containing two variants of an allele or marker. These markers are present in diploid form, meaning there are maternal and paternal pairs; however, they may carry different alleles and variants of the same gene. Autosomal DNA examination is suitable for determining familial relationships through bloodlines. The likelihood of establishing a direct familial relationship increases with the number of markers tested and the size of the sections examined. The autosomes are commonly used in forensic science for the identification and assignment of skeletal elements in disturbed burial sites, reconstruction of historic genealogies, and families.
Nuclear DNA is typed on the Illumina platform. Besides ready-to-use commercial kits, we have been developing in-house protocols for Next-Generation Sequencing (NGS) analyses, specifically targeting forensically and population genetically relevant markers. Additionally, we perform shotgun sequencing on the indexed DNA libraries.
The results are evaluated by programs and bioinformatics tools which are used by international laboratories. Link: Software | David Reich Lab (harvard.edu). Following publication, raw and evaluated DNA data become available in international online databases (ENA, NCBI) and as online supplementary materials accompanying the publications.
Y-chromosome DNA
 In addition to autosomes, the nuclear genome also contains sex chromosomes (X and Y). The Y-chromosome is inherited through the paternal lineage and has relatively few coding regions and a high mutation rate. Most part of it (95%, a 24 Megabase pair long section) undergo recombination with the X-chromosome. Analyzing its markers enables the tracing of paternal lineages and past male-specific population genetic events. The multiplex analysis techniques of Y chromosomal SNPs (single nucleotide polymorphisms) and STRs (short tandem repeats) play significant roles in our studies. Since 2015, we have also employed next-generation sequencing techniques in these analyses.
In addition to autosomes, the nuclear genome also contains sex chromosomes (X and Y). The Y-chromosome is inherited through the paternal lineage and has relatively few coding regions and a high mutation rate. Most part of it (95%, a 24 Megabase pair long section) undergo recombination with the X-chromosome. Analyzing its markers enables the tracing of paternal lineages and past male-specific population genetic events. The multiplex analysis techniques of Y chromosomal SNPs (single nucleotide polymorphisms) and STRs (short tandem repeats) play significant roles in our studies. Since 2015, we have also employed next-generation sequencing techniques in these analyses.
Analysis of mitochondrial DNA (mtDNA)
 The mitochondria generate the cell's supply of adenosine triphosphate (ATP), which serves as a source of chemical energy. Mitochondria have their own genome (mtDNA) with a circular configuration. In contrast to nuclear chromosomal DNA, which is present in each somatic cell as a double set, mtDNA exists only as a single copy. It is primarily inherited through the maternal lineage because female mitochondria predominantly contribute to the newly developing individual's genetic makeup, and all members of a maternal lineage share the same mtDNA ancestry. Since several hundred mitochondria are found in a single cell, their DNA markers can provide valuable information on maternal relationships, population genetics, and population history. Our primary focus was on the Hypervariable Region I (HVR-I) of the mitochondrial genome, which is the most relevant and well-studied part of mtDNA in population genetic studies. We complemented this analysis by typing several polymorphisms in the coding region of mtDNA to validate our data and confirm certain haplogroup classifications. Since 2015, PCR-based methods have gradually been replaced by whole mitochondrial genome sequencing using target capture protocols and the Illumina MiSeq sequencer. DNA library preparation and capture protocols have also been optimized for the Biomek i5 robot, thereby increasing the laboratory's sample processing capacity.
The mitochondria generate the cell's supply of adenosine triphosphate (ATP), which serves as a source of chemical energy. Mitochondria have their own genome (mtDNA) with a circular configuration. In contrast to nuclear chromosomal DNA, which is present in each somatic cell as a double set, mtDNA exists only as a single copy. It is primarily inherited through the maternal lineage because female mitochondria predominantly contribute to the newly developing individual's genetic makeup, and all members of a maternal lineage share the same mtDNA ancestry. Since several hundred mitochondria are found in a single cell, their DNA markers can provide valuable information on maternal relationships, population genetics, and population history. Our primary focus was on the Hypervariable Region I (HVR-I) of the mitochondrial genome, which is the most relevant and well-studied part of mtDNA in population genetic studies. We complemented this analysis by typing several polymorphisms in the coding region of mtDNA to validate our data and confirm certain haplogroup classifications. Since 2015, PCR-based methods have gradually been replaced by whole mitochondrial genome sequencing using target capture protocols and the Illumina MiSeq sequencer. DNA library preparation and capture protocols have also been optimized for the Biomek i5 robot, thereby increasing the laboratory's sample processing capacity.